- The ADA recognizes the use of fluoride and community water fluoridation as safe and effective in preventing tooth decay for both children and adults. For more information, please visit the ADA Fluoride in Water resource page.
- Fluoride is a mineral that is found in all natural water sources and is the ionic form of the trace element fluorine, which is commonly found in the environment; fluorine reaches water sources by leaching from soil and rocks into groundwater.
- When used as directed or within the context of community water fluoridation programs, fluoride is a safe and effective agent that can be used to prevent and control dental caries.
- Fluoride can be delivered topically and systemically. Topical fluorides strengthen teeth already present in the mouth, making them more decay resistant, while systemic fluorides are those that are ingested and become incorporated into forming tooth structures. Systemic fluorides also provide topical protection because fluoride is present in saliva, which continually bathes the teeth.
- Self-applied topical fluorides include toothpastes, mouthrinses, and gels. Professionally applied topical fluorides include higher-strength rinses, gels, and foams; fluoride varnishes; and silver diamine fluoride.
- Community water fluoridation is the process of adjusting the fluoride content of fluoride-deficient water to the recommended level for optimal dental health, which is currently recommended at 0.7 parts fluoride per million parts water.
- Many bottled waters on the market do not contain optimal levels of fluoride. In addition, some types of home water treatment systems (e.g., reverse osmosis and distillation systems) can reduce the fluoride levels in water supplies, potentially decreasing the decay-preventive effects of optimally fluoridated water; however, carbon/charcoal filtration systems do not remove fluoride.
- Fluoride supplements can be prescribed for children ages 6 months to 16 years who are at high risk for tooth decay and whose primary drinking water has a low fluoride concentration.
- A potential risk of fluoride use is the development of fluorosis, which may occur when excess levels of fluoride are ingested during tooth development. Fluorosis varies in appearance from white striations to stained pitting of enamel.
Fluoride: Topical and Systemic Supplements
Key Points
Fluoride is a mineral that is found in all natural water sources.1 Fluoride is the ionic form of the trace element fluorine. Fluorine is commonly found in the environment, and reaches water sources by leaching from soil and rocks into groundwater.1
When used as directed or within the context of community water fluoridation programs, fluoride is a safe and effective agent that can prevent and control dental caries.2, 3 The process of caries is multifactorial and, over time, can culminate in localized destruction of hard dental tissues by the weak acids produced by bacterial carbohydrate fermentation.3-5 Fluoride remineralizes the calcium hydroxyapatite structure in enamel by forming calcium fluorapatite, which is more resistant to acid attacks.1 The remineralization effect of fluoride can both reverse the early decay process as well as create a tooth surface that is more resistant to decay.5
Fluoride can be delivered topically and systemically.1, 3 Topical fluorides strengthen teeth already present in the mouth, making them more decay resistant.3, 5, 6 Topical fluorides encourage remineralization of enamel, and also inhibit bacterial metabolism, reducing the growth of plaque bacteria.1 Modes of topical fluoride delivery include toothpastes, gels, mouthrinses, and professionally applied fluoride therapies.1
Systemic fluorides are those that are ingested and become incorporated into forming tooth structures.1, 5 Systemic fluorides can also confer topical protection because fluoride is present in saliva, which continually bathes the teeth.1 Modes of systemic fluoride delivery include water fluoridation or dietary fluoride supplements in the form of tablets, drops, or lozenges.2
Self-Applied
Fluoride Toothpaste. Fluoride-containing toothpaste is the most commonly used form of self-applied fluoride worldwide.3 Fluoride in toothpaste is taken up directly by the dental plaque and demineralized enamel and also increases the concentration of fluoride in saliva.2, 3 Brushing with fluoride toothpaste increases the fluoride concentration in saliva 100- to 1,000-fold; this concentration returns to baseline levels within 1 to 2 hours.3 Fluoride toothpaste makes up more than 95% of toothpaste sales in the U.S.2The American Dental Association recommends use of a fluoride toothpaste displaying the ADA Seal of Acceptance. Fluoride toothpastes available over the counter in the U.S. generally contain a fluoride concentration of 1,000 to 1,500 ppm.2, 5, 6 Prescription-strength fluoride toothpastes contain 5,000 ppm fluoride as sodium fluoride.2, 6 In the U.S., the active ingredient in fluoride-containing toothpastes can be sodium fluoride, sodium monofluorophosphate, or stannous fluoride.5, 6
For most people (children, adolescents, and adults) brushing twice a day with a fluoride toothpaste—when you get up in the morning and before going to bed—is recommended.2 Children’s brushing should be supervised to ensure that they use the appropriate amount of toothpaste. For children younger than 3 years, parents and caregivers should begin brushing children’s teeth as soon as they begin to come into the mouth by using fluoride toothpaste in an amount described as no more than a smear or alternatively as the size of a grain of rice.3 For children 3 to 6 years of age, parents and caregivers should dispense no more than a pea-sized amount of fluoride toothpaste.3
Fluoride Mouthrinse or Gels. Fluoride mouthrinse is a concentrated solution intended for daily or weekly use and designed to be rinsed and spit out.2 The most common fluoride compound used in mouthrinse is sodium fluoride.2 The fluoride from mouthrinse is retained in dental plaque and saliva and helps prevent tooth decay.2, 3 Over-the-counter solutions of 0.05% sodium fluoride (230 ppm fluoride) for daily rinsing are available for use by persons older than 6 years of age;2, 5, 6 use in persons younger than 6 years of age is not recommended because of the risk of fluorosis if the rinse is swallowed repeatedly.3, 6 Higher strength mouthrinses (e.g., 0.2% neutral sodium fluoride to be used once a week) for those at high risk of tooth decay must be prescribed by a dentist or physician.2 Solutions of 0.2% sodium fluoride (920 ppm fluoride) are also used in supervised, school-based weekly rinsing programs.2, 3, 5
There are also self-applied gel formulations of sodium fluoride (1.1% [5,000 ppm] sodium fluoride) or stannous fluoride (0.15% [1,000 ppm] fluoride) available by prescription for home use.2, 5
Professionally Applied
Fluoride Mouthrinse, Gels, or Foams. Professionally applied fluorides are in the form of a gel, foam or rinse, and are applied by a dental professional during dental visits.2 These fluorides are more concentrated than the self-applied fluorides (e.g., 1.23% fluoride ion [12,300 ppm]), and therefore are not needed as frequently.
Because an early study7 reported that fluoride uptake by dental enamel increased in an acidic environment, fluoride gel is often formulated to be highly acidic (pH of approximately 3.0).3 Products available in the U.S. include gels of acidulated phosphate fluoride (1.23% [12,300 ppm] fluoride), as 2% neutral sodium fluoride products (containing 9,000 ppm fluoride), and as gels or foams of sodium fluoride (0.9% [9,040 ppm] fluoride).2, 5 In a dental office, fluoride gel is generally applied for 1 to 4 minutes.2, 5 Home use follows instructions provided in the package insert or as instructed by a dentist or physician.2 These higher strength products, if used in the home, must be prescribed by a dentist or physician.
Because these applications are relatively infrequent, generally at 3- to 12-month intervals, fluoride gel poses little risk for dental fluorosis, even among patients younger than 6 years of age.2, 3 Routine use of professionally applied fluoride gel or foam likely provides benefit only to persons at high risk for tooth decay, especially those who do not consume fluoridated water and brush daily with fluoride toothpaste.2
Fluoride-Containing Prophylaxis Paste. Fluoride-containing paste is routinely used during dental prophylaxis. The abrasive paste, which contains 4,000 to 20,000 ppm fluoride, might restore the concentration of fluoride in the surface layer of enamel removed by polishing, but it is not an adequate substitute for fluoride gel or varnish in treating persons at high risk for dental caries.3 Fluoride prophylaxis paste alone is not considered by the U.S. Food and Drug Administration (FDA) or ADA an effective method to prevent dental caries.3, 8
Fluoride Varnish. Varnishes are available as sodium fluoride (2.26% [22,600 ppm] fluoride) or difluorsilane (0.1% [1,000 ppm] fluoride) preparations.2, 5, 6 A typical application requires 0.2 to 0.5 mL, resulting in a total fluoride ion application of approximately 5 to 11 mg.5
High-concentration fluoride varnish is painted by dental or other health care professionals directly onto the teeth and sets when it comes into contact with saliva.2, 5, 6 Fluoride varnish is not intended to adhere permanently; this method holds a high concentration of fluoride in a small amount of material in close contact with the teeth for several hours.2 Varnishes must be reapplied at regular intervals with at least 2 applications per year needed for sustained benefit.2 Although it is not currently cleared for marketing by the FDA as an anticaries agent, fluoride varnish has been widely used for this purpose in Canada and Europe since the 1970s.2, 3 Studies conducted in Canada and Europe have reported that fluoride varnish is as effective in preventing tooth decay as professionally applied fluoride gel.2 The U.S. Preventive Services Task Force recommends the clinical application of fluoride varnish to the primary teeth of all infants and children starting at the age of primary tooth eruption.9 The recommendation is given a “B” grade, indicating that there is high certainty that the net benefit of the intervention is moderate or there is moderate certainty that the net benefit is moderate to substantial.10
According to the Centers for Disease Control and Prevention (CDC), there is no published evidence to indicate that professionally applied fluoride varnish is a risk factor for dental fluorosis, even among children younger than 6 years of age.2 Proper application technique reduces the possibility that a patient will swallow varnish during its application and limits the total amount of fluoride swallowed as the varnish wears off the teeth over a period of hours.11
Silver Diamine Fluoride. Silver diamine fluoride (SDF) is a colorless liquid that at pH 10 is 24.4% to 28.8% (weight/volume) silver and 5.0% to 5.9% fluoride.12 The FDA has classified SDF as a Class II medical device and it is cleared for use in the treatment of tooth sensitivity, which is the same type of clearance as fluoride varnish, and must be professionally applied. Although some products are commercially available in other countries, currently, Advantage Arrest™ (Elevate Oral Care, L.L.C.) and Riva Star™ (SDI, Inc.) are the only commercially available SDF products for dental use in the U.S.13 There have been reports of the use of SDF in caries control and management, although it is not specifically labeled for use for this indication (i.e., “off-label use”). Likely a result of its fluoride content, when applied to a carious lesion, SDF has been shown to lower caries risk of the adjacent tooth surface.14 SDF has also shown efficacy in management of root caries in the elderly.15-17 It likely has additional applicability as an interim approach for managing problematic caries in individuals currently unable to tolerate more involved dental treatment.18
Single application of SDF has been reported to be insufficient for sustained benefit.19 Its potential downsides include a reportedly unpleasant metallic taste, potential to irritate gingival and mucosal surfaces, and the characteristic black staining of the tooth surfaces to which it is applied.13
Systemic fluorides such as community water fluoridation and dietary fluoride supplements are effective in reducing tooth decay. These fluorides provide topical as well as systemic protection because fluoride is present in the saliva.
Water Fluoridation
Fluoride is present naturally in all water sources.1 Community water fluoridation is the process of adjusting the fluoride content of fluoride-deficient water to the recommended level for optimal dental health, which is currently recommended at 0.7 parts fluoride per million parts water.20, 21 Water fluoridation is an effective and inexpensive means of obtaining the fluoride necessary to prevent tooth decay.3 Studies show that water fluoridation continues to be effective in reducing tooth decay by 20% to 40% in children and adults, even in the era of widespread availability of fluoride from other sources, such as fluoride toothpaste.22 While water fluoridation is an extremely effective and inexpensive means of obtaining the fluoride necessary for optimal tooth decay prevention, not everyone lives in a community with a centralized, public or private water source that can be fluoridated.22 For those individuals, fluoride is available in other forms.
There are several ways to determine the concentration of fluoride in the water supply.3, 22 If water comes from a public or community water supply, contact the local water supplier to determine the fluoride level. Local, county or state health departments can also be a resource for this information. The U.S. Environmental Protection Agency's (EPA) website for water quality reports (called Consumer Confidence Reports) provides information, as does the U.S. Centers for Disease Control and Prevention's (CDC) fluoridation website, "My Water's Fluoride." The CDC website lists fluoridation status by water system for those states that have provided information.
If the water source is a private well, it will need to be tested and the results obtained from a certified laboratory.22 The local or state health department will have water sample testing information. Although the EPA does not have the authority to regulate private drinking water wells, the agency recommends that private well water be tested every year. And although the EPA does not specifically recommend testing private wells for fluoride levels, health professionals will need this information before consideration of prescription of dietary fluoride supplements or to counsel patients about alternative water sources to reduce the risk of fluorosis if the fluoride levels are above 2 ppm.
The majority of bottled waters on the market contain less than 0.3 ppm fluoride, which is less than the optimal level of fluoride.3, 11 The FDA announced in April 2019 that it is proposing to revise the quality standard for bottled water to state that bottled water to which fluoride is added by the manufacturer may not contain fluoride that exceeds 0.7 milligrams per liter (0.7 ppm).23, 24 If finalized, the proposed rule would amend the allowable levels of fluoride in domestically packaged and imported bottled water to which fluoride is added.
Some types of home water treatment systems (e.g., reverse osmosis and distillation systems) can reduce the fluoride levels in water supplies, potentially decreasing the decay-preventive effects of optimally fluoridated water; however, carbon/charcoal filtration systems do not remove fluoride.11
Dietary Fluoride Supplements
Fluoride supplements can be prescribed for children ages 6 months to 16 years who are at high risk for tooth decay and whose primary drinking water has a low fluoride concentration.2, 25 Tablets and lozenges are manufactured with 1.0, 0.5, or 0.25 mg fluoride.2, 3 Most supplements contain sodium fluoride as the active ingredient.2 To maximize the topical effect of fluoride, tablets and lozenges are intended to be chewed or sucked for 1–2 minutes before being swallowed;2, 3, 5 for infants, supplements are available as a liquid and used with a dropper.3 Dosing is based on the natural fluoride concentration of the child's drinking water and the age of the child (see Table).5, 25
All dietary fluoride supplements must be prescribed by a dentist or physician.2 For children aged younger than 6 years, health care providers should weigh the risk for tooth decay without fluoride supplements, the decay prevention offered by supplements, and the potential for dental fluorosis.2 Consideration of the child's other sources of fluoride, especially drinking water, is essential in determining this balance.3, 5 Parents and caregivers should be informed of both the benefit of protection against tooth decay and the potential risk of dental fluorosis.2 The U.S. Preventive Services Task Force recommends the clinical use of oral fluoride supplementation starting at age 6 months through 5 years for children whose water supply is deficient in fluoride.9 The recommendation is given a “B” grade, indicating that there is high certainty that the net benefit of the intervention is moderate or there is moderate certainty that the net benefit is moderate to substantial.10
Table. Fluoride Supplement (Tablets and Drops) Dosage Schedule 2010 (Approved by the American Dental Association Council on Scientific Affairs)25
| Age | Fluoride Ion Level in Drinking Water (ppm)* | ||
| <0.3 | 0.3-0.6 | >0.6 | |
| Birth-6 months | None | None | None |
| 6 months-3 years | 0.25 mg/day** | None | None |
| 3-6 years | 0.50 mg/day | 0.25 mg/day | None |
| 6-16 years | 1.0 mg/day | 0.50 mg/day | None |
| *1.0 part per million (ppm) = 1 milligram per liter (mg/L) **2.2 mg sodium fluoride contains 1 mg fluoride ion |
|||
Important Considerations When Using Dosage Schedule:25
- If fluoride level is unknown, drinking water should be tested for fluoride content before supplements are prescribed. For testing of fluoride content, contact the local or state health department.
- All sources of fluoride should be evaluated with a thorough fluoride history.
- Patient exposure to multiple water sources may complicate proper prescribing.
- Ingestion of higher than recommended levels of fluoride by children has been associated with an increased risk of mild dental fluorosis in developing, unerupted teeth.
- To obtain the benefits from fluoride supplements, long-term compliance on a daily basis is required.
It is important to note that fluoridated water may be consumed from sources other than the home water supply, such as the workplace, school and/or day care, bottled water, filtered water and from processed beverages and foods prepared with fluoridated water. For this reason, dietary fluoride supplements should be prescribed by carefully following the recommended dosage schedule. Dietary fluoride supplements are not recommended for children residing in a community with adequate levels of fluoride in the water supply.
The ADA’s dietary fluoride supplement recommendations remain unchanged in light of the new guidelines for community water fluoridation in the U.S. released in April 2015 by the U.S. Public Health Service.21 The recommendation for fluoride levels in drinking water was reconsidered in 2015 when it was determined that 0.7 milligrams of fluoride per liter of water (0.7 ppm) was optimal. The new recommendation, which was supported by the ADA, does not change the ADA Council on Scientific Affairs’ systematic review, clinical recommendation and chairside guidefor the use of dietary fluoride supplements that were released in 2010.
In 2013, the ADA Center for Evidence-Based Dentistry and a panel of experts convened by the ADA Council on Scientific Affairs developed clinical recommendations for use of professionally applied or prescription-strength, home-use topical fluorides for caries prevention in patients at high risk of developing caries.8 The Panel evaluated sodium, stannous and acidulated phosphate fluoride for professional and prescription-strength home-use, including varnishes, gels, foams, mouthrinses and prophylaxis pastes. The Panel did not include over-the-counter products, slow-release delivery devices, dental materials that release fluorides or products containing sodium monofluorophosphate, silver diamine fluoride, and titanium tetrafluoride in its report. The Panel included 71 trials from 82 articles in the review and assessed the efficacy of various topical fluoride caries-preventive agents.
Based on the literature review and consensus, the Panel recommended the following professionally applied or prescription-strength, home-use topical fluorides for caries prevention in patients at elevated risk:
- 2.26% fluoride varnish or 1.23% fluoride (acidulated phosphate fluoride) gel, or a prescription-strength, home-use 0.5% fluoride gel or paste or 0.09% fluoride mouthrinse for patients 6 years or older
- Only 2.26% fluoride varnish was recommended for children younger than 6 years
The strengths of the recommendations for the recommended products varied from “in favor” to “expert opinion for.” As stated in the report, as part of the evidence-based approach to care, these clinical recommendations “should be integrated with the practitioner’s professional judgment and the patient’s needs and preferences.” The Panel also determined that patients at low risk of developing caries may receive additional benefit from application of topical fluorides beyond that achieved from their daily use of over-the-counter fluoridated toothpaste and consumption of fluoridated water.
In 2018, the ADA Center for Evidence-Based Dentistry conducted a systematic review and network meta-analysis26informing a clinical practice guideline27 on nonrestorative treatments for carious lesions. The expert panel formulated 11 clinical recommendations, each specific to lesion type (i.e., cavitated, noncavitated), tooth surface (i.e., coronal, root surface [in adults]) and dentition (i.e., primary or permanent). The panel provided recommendations for the use of the most effective treatment options, including 38% silver diamine fluoride, sealants, 5% sodium fluoride varnish, 1.23% acidulated phosphate fluoride gel, and 5,000 parts per million fluoride (1.1% sodium fluoride) toothpaste or gel, among others. The panel also provided a recommendation against the use of 10% casein phosphopeptide–amorphous calcium phosphate. The chairside guides for primary and permanent dentition are available for download, and clinicians may also consult the online tool for personalized clinical recommendations based on the clinical parameters of the lesion.
A potential risk of fluoride use is the development of fluorosis, which may result from fluoride ingestion during tooth development.6 Fluorosis of permanent teeth occurs when an excess quantity of fluoride is ingested for a sufficient period of time during the time that tooth enamel is being mineralized.6 The level of fluoride intake between the ages of about 15 and 30 months is thought to be most critical for the development of fluorosis of the maxillary central incisors.11 The mechanisms by which fluoride modifies tooth development are not fully understood; but may result from alterations in protein metabolism disrupting the crystal organization in the developing tooth.28
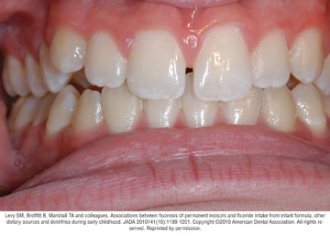
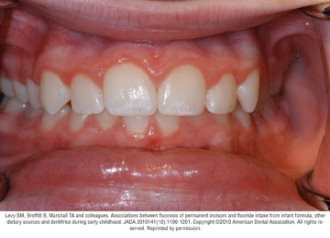
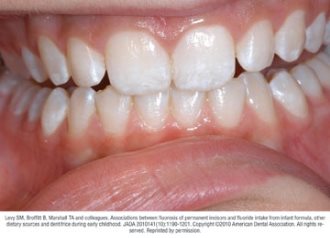
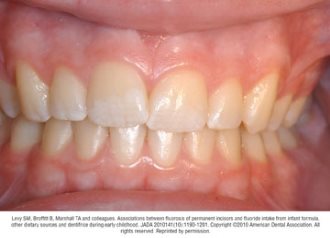
Fluorosis varies in appearance from white striations to stained pitting of enamel11 (see Figures).6 Enamel fluorosis occurs only when primary and permanent teeth are developing. Once teeth erupt, they cannot develop enamel fluorosis.6 Excess fluoride exposure can be minimized by using the recommended amount of toothpaste and by storing toothpaste where young children cannot access it without parental assistance.6 Parents should also be strongly advised to supervise their child’s use of fluoride toothpaste to avoid overuse or ingestion.6
Figures. Four typical cases of mild fluorosis, seen in children participating in the Iowa Fluoride Study28
|
|
|
|
|
|
|
|
Community-Based Topical Fluoride Programs
(Trans.2014:507)
Resolved, the American Dental Association recognizes that community-based topical fluoride programs are safe and efficacious in reducing dental caries.
American Dental Association
Adopted 2014
Bottled Water, Home Water Treatment Systems and Fluoride Exposure
(Trans.2002:390; 2013:342; 2021:327))
Resolved, that in order to ensure optimal fluoride intake, the American Dental Association supports actions by its members to educate their patients regarding the level of fluoride in bottled water and the possible removal of fluoride by some home water treatment systems, and be it further
Resolved, that the American Dental Association urges its members to inquire about their patients’ primary and secondary water source as part of the health history, and be it further
Resolved, that the American Dental Association supports the labeling of bottled water with the fluoride concentration of the product and company contact information including address and telephone number and website, and be it further
Resolved, that the American Dental Association urges its members and the public to refer to the International Bottled Water Association’s “List of Brands Containing Fluoride,” and be it further
American Dental Association
Adopted 2002; Amended 2013; Amended 2021
Groundwater With Natural Levels of Fluoride Higher Than 2.0 Parts Per Million
(Trans.1999:921)
Resolved, that the American Dental Association urge state dental societies to continue efforts to educate professionals and consumers about the role of fluoride in community oral health, and be it further
Resolved, that the Association urge state dental societies to encourage state and local dental public health and drinking water authorities to identify the state’s groundwater sectors with natural fluoride levels that exceed 2.0 parts per million, and be it further
Resolved, that the Association encourage state and local dental societies to communicate with local health and drinking water authorities regarding standards for fluoride levels, and be it further
Resolved, that the Association urge dentists to become familiar with the water fluoride concentrations in their area of practice that exceed 2.0 parts per million and provide appropriate counseling to parents and caregivers of young children to reduce the risk of dental fluorosis in permanent teeth, and be it further
Resolved, that the Association encourage dentists to educate pediatric health care workers about groundwater sectors and water systems with fluoride levels that exceed 2.0 parts per million so that parents and caregivers of young children receive appropriate counseling to reduce the risk of dental fluorosis in permanent teeth.
American Dental AssociationAdopted 1999
Operational Policies and Recommendations Regarding Community Water Fluoridation
(Trans.1997:673; 2015:273)
- The Association endorses community water fluoridation as a safe, beneficial and cost-effective and socially equitable public health measure for preventing dental caries in children and adults.
- The Association supports the fluoridation of community water systems as recommended by the U.S. Public Health Service.
- The Association urges individual dentists and dental societies to exercise leadership in all phases of activity which lead to the initiation and continuation of community water fluoridation, including making scientific knowledge and resources available to the community and collaborating with state and local agencies.
- The Association encourages governmental, philanthropic and other entities to make funding available to communities seeking to initiate and/or maintain community water fluoridation.
- The Association supports the following actions to maintain the quality of national community water fluoridation and its infrastructure:
- performance of periodic assessments of community water fluoridation infrastructure needs by appropriate state agencies;
- allocation of needed resources to or by appropriate state agencies to upgrade and maintain the fluoridation infrastructure;
- and observance of the standards established by the appropriate state agencies related to engineering and administrative recommendations for water fluoridation in accordance with guidance issued by the Centers for Disease Control and Prevention.
American Dental Association
Adopted 1997; Amended 2015
Policy on Fluoridation of Water Supplies
(Trans.1950:224; 2015:274)
Resolved, that in the interest of public health, the American Dental Association recommends the fluoridation of community water systems in accordance with the standards established by the appropriate authority, and be it further
Resolved, that the American Dental Association supports ongoing research on the safety and effectiveness of community water fluoridation.
American Dental Association
Adopted 1950; Amended 2015
- McGrady MG, Ellwood RP, Pretty IA. Why fluoride? Dent Update 2010;37(9):595-8, 601-2.
- Centers for Disease and Prevention. Other Fluoride Products. U.S. Department of Health and Human Services. Accessed June 14, 2023.
- Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 2001;50(Rr-14):1-42.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51-9.
- Adair SM. Evidence-based use of fluoride in contemporary pediatric dental practice. Pediatr Dent 2006;28(2):133-42; discussion 92-8.
- Clark MB, Slayton RL. Fluoride use in caries prevention in the primary care setting. Pediatrics 2014;134(3):626-33.
- Brudevold F, Savory A, Gardner DE, Spinelli M, Speirs R. A study of acidulated fluoride solutions. I. In vitro effects on enamel. Arch Oral Biol 1963;8:167-77.
- Weyant RJ, Tracy SL, Anselmo TT, et al. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc 2013;144(11):1279-91.
- U.S. Preventive Services Task Force. Final Recommendation Statement: Dental Caries in Children from Birth Through Age 5 Years: Screening. December 2016. Accessed May 9, 2023.
- U.S. Preventive Services Task Force. Grade Definitions. October 2018. Accessed July 15, 2021.
- Levy SM. An update on fluorides and fluorosis. J Can Dent Assoc 2003;69(5):286-91.
- Mei ML, Chu CH, Lo EC, Samaranayake LP. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent 2013;23(4):279-85.
- Mei ML, Lo EC, Chu CH. Clinical Use of Silver Diamine Fluoride in Dental Treatment. Compend Contin Educ Dent 2016;37(2):93-8; quiz100.
- Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res 2005;84(8):721-4.
- Li R, Lo EC, Liu BY, Wong MC, Chu CH. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J Dent 2016.
- Zhang W, McGrath C, Lo EC, Li JY. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res 2013;47(4):284-90.
- Hendre AD, Taylor GW, Chavez EM, Hyde S. A systematic review of silver diamine fluoride: Effectiveness and application in older adults. Gerodontology 2017.
- Giusti L, Steinborn C, Steinborn M. Use of silver diamine fluoride for the maintenance of dental prostheses in a high caries-risk patient: A medical management approach. J Prosthet Dent 2017.
- Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: rationale, indications and consent. J Calif Dent Assoc 2016;44(1):16-28.
- Federal Register. Proposed HHS Recommendation for Fluoride Concentration in Drinking Water for Prevention of Dental Caries (Volume 76, Number 9; pages 2383-2388). U.S. Department of Health and Human Services 2011. Accessed June 14, 2023.
- U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep 2015;130(4):318-31.
- ADA. Fluoridation Facts: American Dental Association; 2018.
- U.S. Food and Drug Administration. FDA Constituent Update: FDA Announces Proposed Ruling on Fluoride in Bottled Water. U.S. Department of Health and Human Services 2019. Accessed June 14, 2023.
- U.S. Food and Drug Administration. FDA In Brief: FDA proposes updated standards for fluoride added to bottled water to maximize health benefits while avoiding excess exposure. U.S. Department of Health and Human Services 2019. Accessed June 14, 2023.
- Rozier RG, Adair S, Graham F, et al. Evidence-based clinical recommendations on the prescription of dietary fluoride supplements for caries prevention: a report of the American Dental Association Council on Scientific Affairs. J Am Dent Assoc 2010;141(12):1480-9.
- Urquhart O, Tampi MP, Pilcher L, et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res 2018:22034518800014.
- Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837-49.e19.
- Levy SM, Broffitt B, Marshall TA, Eichenberger-Gilmore JM, Warren JJ. Associations between fluorosis of permanent incisors and fluoride intake from infant formula, other dietary sources and dentifrice during early childhood. J Am Dent Assoc 2010;141(10):1190-201.
- Fluoride in Water
- ADA Fluoridation Video
- ADA Store: Fluoridation Facts (Catalog Item J120BT)
- Chairside Guide: Dietary Fluoride Supplements: Evidence-based Clinical Recommendations
- Search JADA for articles related to fluoridation
- ADA Library Services
- PPR, Vol 12, Iss 2: Fluoride Analysis of Fluoride Varnish and SDF (ADA log-in required)
- PPR, Vol 10, Iss 3: Fluoride Analysis of Fluoride Varnish (ADA log-in required)
- For the Patient: Sources of Fluoride (2023)
- MouthHealthy
- Video: Fluoride: The Superhero of Cavity Fighting
Centers for Disease Control and Prevention:
Topic last updated: June 14, 2023
Prepared by:
Research Services and Scientific Information, ADA Library & Archives.
Disclaimer
Content on the Oral Health Topics section of ADA.org is for informational purposes only. Content is neither intended to nor does it establish a standard of care or the official policy or position of the ADA; and is not a substitute for professional judgment, advice, diagnosis, or treatment. ADA is not responsible for information on external websites linked to this website.